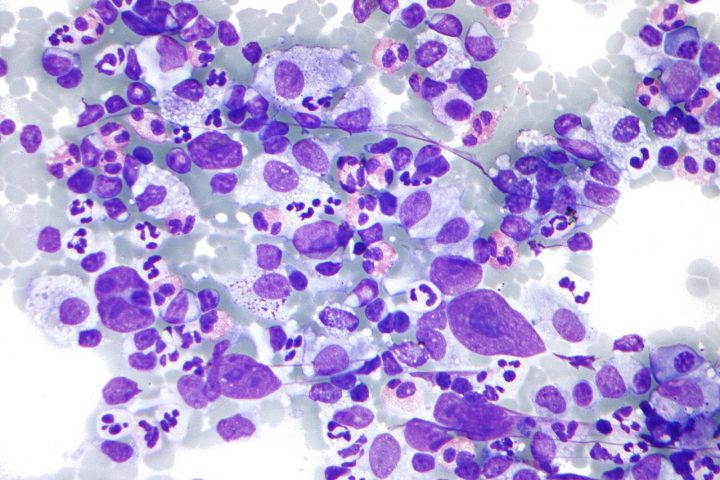
On August 28, 2023, the U.S. Food and Drug Administration approved luspatercept-aamt (Reblozyl) for the treatment of anemia in adult patients with very low-
Written by Dr. Chepsy Philip from Believers Church Medical College Hospital, Tiruvalla, Kerala, India KEYNOTE-087 is a multicentre, single-arm, multicohort, nonrandomized, phase
By Dr. Lei Deng University of Washington & Fred Hutch Watch Dr. Lei Deng’s overview of the KEYNOTE-671
By Akshat Jain MD, MPH Hematology Oncology and Cellular Therapy Faculty Department of Pediatrics & Clinical Medicine Loma Linda University School of
By Dr. Anish Shah Bronx-Lebanon Hospital On August 14, 2023, the U.S. Food and Drug Administration approved HEPZATO KIT – which
By Dr. Anish Shah Bronx-Lebanon Hospital On August 14, 2023, the U.S. Food and Drug Administration granted accelerated approval to Elranatamab-bcmm
By Dr. Anish Shah Bronx Lebanon Hospital On August 11, 2023, the U.S. Food and Drug Administration granted regular approval to
By Dr. Anish Shah Bronx Lebanon Hospital On August 9, 2023, the U.S. Food and Drug Administration approved talquetamab-tgvs (Talvey, Janssen
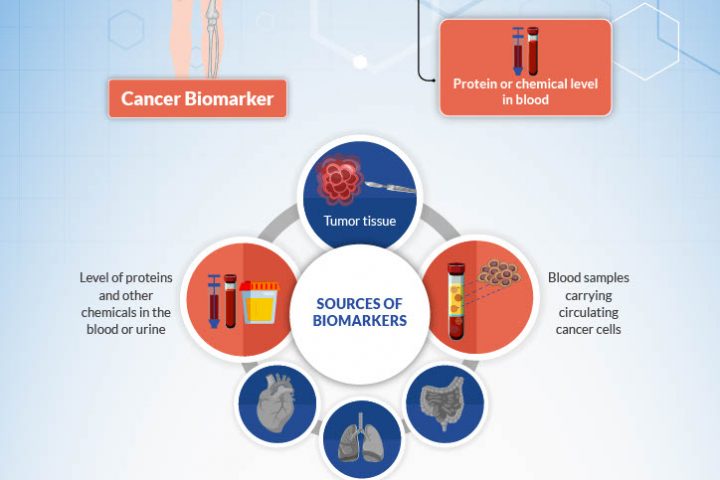
By Dr. Dipesh Uprety Karmanos Cancer Institute & Dr. Howard (Jack) West City of Hope What is a biomarker, and
By Dr. Anish Shah Bronx-Lebanon Hospital On August 9, 2023, the U.S. Food and Drug Administration granted regular approval to pralsetinib