On April 3, 2023, the U.S. Food and Drug Administration approved enfortumab vedontin-ejfv with pembrolizumab for patients with locally advanced or metastatic
On March 29, 2023, the U.S. Food and Drug Administration approved pembrolizumab for adult and pediatric patients with unresectable or metastatic solid
By Dr. Shipra Gandhi Roswell Park Comprehensive Cancer Center Endocrine therapy is the mainstay of the management of hormone receptor (HR)
On March 22, 2023, the U.S. Food and Drug Administration granted accelerated approval to retifanlimab-dlwr — a humanized monoclonal antibody that targets
The International Innovation Grant will Fund Research on the Effectiveness of Community Screening Programs for the Early Detection of Oral Cancers in
By Dr. Luke Fletcher Willamette Valley Cancer Institute & Research Center The Zuma 7 trial is a randomized, open-label multicenter study for
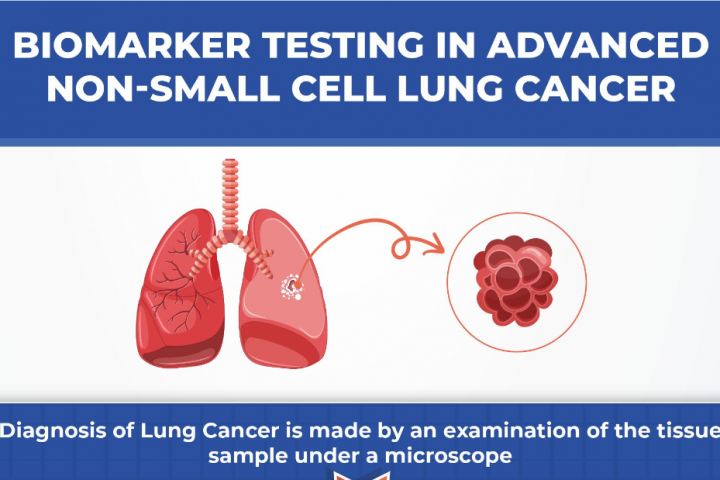
By Dr. Dipesh Uprety Karmanos Cancer Institute
By Dr. Radhika Kulkarni Henry Ford Cancer Institute On March 2nd, 2023, results of the Phase 2 trial, SWOG 1801, (NCT03698019)
On March 16, 2023, the U.S. Food and Drug Administration approved dabrafenib and trametinib for pediatric patients 1 year of age and
By Dr. Dipesh Uprety Karmanos Cancer Institute While immunotherapy is being used religiously for non-oncogene-addicted advanced non-small cell lung cancer, the