By: Dr. Anish Shah Bronx-Lebanon Hospital; Bronx, NY On April 29, 2024, the U.S. Food and Drug Administration granted regular approval to tisotumab vedotin-tftv (Tivdak, Seagen Inc. [now a part of Pfizer Inc.]) for
By: Tiantian Zhang1, Yiqun Han2, Lei Deng3 1. Department of Internal Medicine, University of Central Florida, Pensacola, FL 2. Mayo Clinic, Rochester, MN 3. University of Washington/Fred Hutch Cancer Center, Seattle, WA
MoreBy: Zhiting Tang1, Lei Deng2 1. Unity Hospital, Rochester Regional Health, Rochester, NY2. University of Washington/Fred Hutch Cancer Center, Seattle, WA LAURA: A randomized, double-blind, Phase III study of chemoradiation followed
MoreBy: Dr. Anish Shah Bronx-Lebanon Hospital; Bronx, NY On April 23, 2024, the U.S. Food and Drug Administration granted accelerated approval for tovorafenib (Ojemda, Day One Biopharmaceuticals, Inc.) for relapsed or refractory pediatric low-grade
MoreBy: Dr. Anish Shah Bronx-Lebanon Hospital; Bronx, NY On April 29, 2024, the U.S. Food and Drug Administration granted regular approval to tisotumab vedotin-tftv (Tivdak, Seagen Inc. [now a part of Pfizer Inc.]) for
MoreFDA Approves Lutetium Lu 177 Dotatate for SSTR-Positive Gastroenteropancreatic Neuroendocrine Tumors
By: Dr. Anish Shah Bronx-Lebanon Hospital; Bronx, NY On April 23, 2024, the U.S. Food and Drug Administration granted approval for lutetium Lu 177 dotatate (Lutathera, Advanced Accelerator Applications USA, Inc., a Novartis company)
MoreBy: Dr. Anish Shah Bronx-Lebanon Hospital; Bronx, NY On April 18, 2024, the U.S. Food and Drug Administration granted approval to a potent oral ALK (anaplastic lymphoma kinase) tyrosine kinase inhibitor – Alectinib (Alecensa,
MoreBy: Dr. Anish Shah Bronx-Lebanon Hospital; Bronx, NY On April 22, 2024, the U.S. Food and Drug Administration granted approval for nogapendekin alfa inbakicept-pmln (Anktiva, Altor BioScience, LLC), to be used in conjunction with
MoreBy: Dr. Anish ShahBronx-Lebanon Hospital; Bronx, NY On April 5, 2024, the Food and Drug Administration granted accelerated approval forfam-trastuzumab deruxtecan-nxki (Enhertu, Daiichi Sankyo, Inc.). The approval targetsadults with inoperable or metastatic HER2-positive
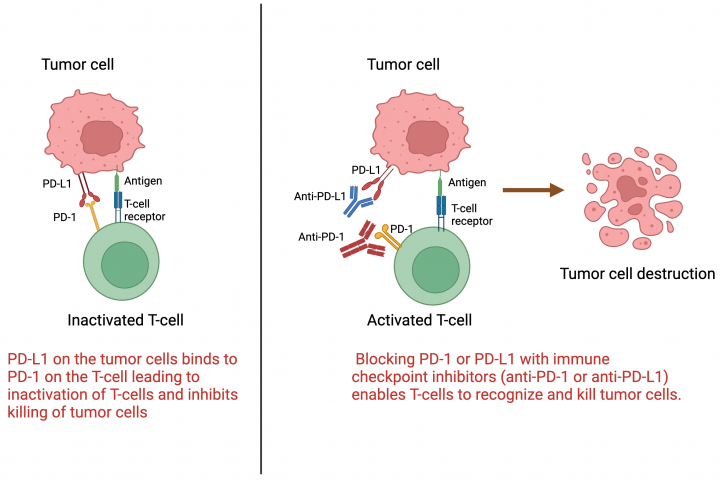
MoreBy: Dr. Arya Mariam Roy Roswell Park Comprehensive Cancer Center What are immune checkpoint inhibitors? Immune checkpoint inhibitors are a type of immunotherapy that harnesses the body’s own immune system to prevent
MoreBy: Dr. Anish Shah Bronx-Lebanon Hospital; Bronx, NY On March 1, 2024, the U.S. Food and Drug Administration approved amivantamab- vmjw (Rybrevant, Janssen Biotech, Inc.) in combination with carboplatin and pemetrexed for the initial
More