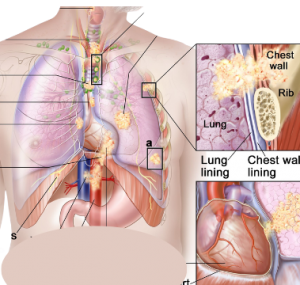
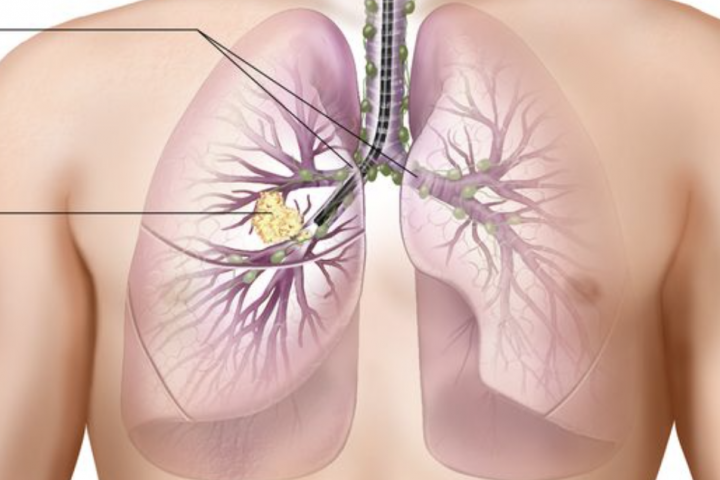
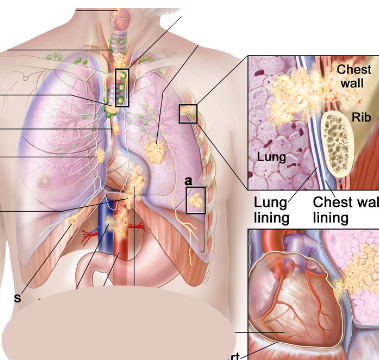
By Dr. Aakash Desai Mayo Clinic KEYNOTE-789: No Significant Efficacy Benefit with Addition of Pembrolizumab to Chemotherapy in TKI-Resistant, EGFR-Mutated Metastatic Nonsquamous NSCLC Key Points: The KEYNOTE-789 trial did not
By: Dr. Anam Kamal Ascension Providence Hospital; Michigan The standard of care for such patients is platinum-based doublet chemotherapy. In May 2021, the FDA granted accelerated approval to Amivantamab for the treatment
MoreBy Dr. Kanak Parmer Texas Tech University Health Sciences Center On October 16,2023 the U.S. Food and Drug Administration approved pembrolizumab for patients with resectable non-small cell lung cancer (NSCLC) tumors
MoreMARIPOSA-2: A Study of Amivantamab and Lazertinib in Combination With Platinum-Based Chemotherapy Compared With Platinum-Based Chemotherapy in Patients With Epidermal Growth Factor Receptor (EGFR)- Mutated Locally Advanced or Metastatic Non- Small Cell
MoreBy Dr. Anam Kamal Ascension Providence Hospital TROPION-Lung01: A randomized, phase-3 study of datopotamab deruxtecan (Dato-DXd; DS-1062) versus docetaxel in previously treated advanced or metastatic non-small cell lung cancer (NSCLC) without actionable
MoreBy Dr. Aakash Desai Mayo Clinic KEYNOTE-789: No Significant Efficacy Benefit with Addition of Pembrolizumab to Chemotherapy in TKI-Resistant, EGFR-Mutated Metastatic Nonsquamous NSCLC Key Points: The KEYNOTE-789 trial did not
MoreAuthor: Lei Deng, MD Roswell Park Comprehensive Cancer Center Live report from ASCO23, Chicago, IL June 6, 2023 LBA9005 Tumor Treating Field (TTFields) therapy with standard of care (SOC) in
More