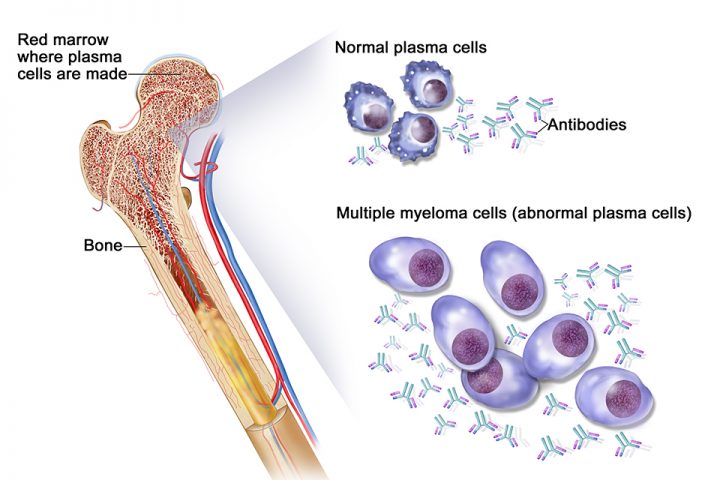
By Dr. Richa Parikh
& Dr. Harsh Parmar
A multicenter retrospective study, which was conducted at 11 academic centers across the United States, reported a comparable efficacy and safety of ‘standard-of-care’ Ide-cel with that observed with the KarMMa trial. These results were demonstrable despite most patients not meeting the eligibility criteria for the KarMMa trial1,2.
196 patients completed leukapheresis with the intent to receive commercial ide-cel. Manufacturing failure was reported in 6% of patients with half of those patients (3%) undergoing successful manufacturing with a repeat attempt. 35% of patients had high-risk cytogenetics (defined as 17p deletion, t(4;14) and t(14;16). 75% of patients had comorbidities that would have made them ineligible to participate in the KarMMa trial. A higher fraction (48%) of patients had extra-medullary disease (vs 39% in the trial), with 44% of patients with penta-refractory disease (vs 26% in the trial). A median time of 47 days was reported from leukapheresis to ide-cel infusion.
A median hospital stay of 9 days (range 5-69 days) was reported. Any grade CRS was observed in 82% of patients, with >= grade 3 CRS in 3% of patients. 71% of patients received tocilizumab, 5% received anakinra and 26% of patients received corticosteroids. >=grade 3 hematological toxicities which persisted longer than 30 days were reported in 60% of patients with neutropenia, 38% with anemia and 59% with thrombocytopenias. 74% of patients received granulocyte colony-stimulating factor, 15% received a thrombopoetin agonist and 5% of patients received an autologous stem cell boost. 54 (34%) patients experienced infections with 31 (20%) bacterial, 24 (16%) viral, and 2(1%) fungal infections. 30 patients (19%) had died at the last follow-up with 20 deaths attributed to disease progression, and 8 (5%) were related to non-relapse mortality.
On univariate analysis, a higher burden of disease including plasmacytosis>=50%, a higher R-ISS, and poor ECOG PS>=2, were associated with a higher CRS grade (>=grade 3) risk. A multivariate analysis was not performed due to lower number of patients experiencing grade 3 CRS.
An ORR of 84% was reported with 42% of patients achieving a CR. 72% of patients achieved MRD negativity (10-5 nucleated cells) in patients with a stringent CR. Median time to response and achieving >=CR was 30 days with a median duration of response of 8.6 months (95% CI 6.6mos-NR).) ORR was reported to be inferior in patients with extra-medullary disease (p=0.03), prior use of anti-BCMA (p=0.04), and penta-refractory status (p=0.009). ORR was higher in patients without prior anti-BCMA therapy compared to those with prior BCMA exposure (87% vs 73%, p=0.04).
At a median follow-up of 6.1 months post-ide-cel, a median PFS of 8.5 months and an OS of 12.5 months were reported respectively. Patients who had prior anti-BCMA therapy had an inferior PFS (median PFS of 3.2 months vs 9.0 months, p= -.00092) as well as OS (7.4 months vs 12.5 months, p=0.01). Most patients received belantamab for their anti-BCMA therapy (n=25). Patients who would have been ineligible for the KarMMa trial had a trend towards inferior PFS (7.6 months vs 9 months, p=0.19).
In conclusion, the safety and efficacy data reported in this study were comparable to those observed in the KarMMa trial, despite most (75%) not meeting the eligibility criteria for the trial. The study also provides insight into potential sequencing strategies for relapsed patients, with consideration towards avoiding anti-BCMA therapy prior to ide-cel. Prior treatment with alkylator therapy has been known to be associated with poor manufacturing, which should also be avoided immediately prior to a planned leukapheresis for ide-cel3. A selinexor-based regimen can be considered prior to anti-BCMA CAR-T, as it may have a favorable effect on T cells as reported in a study conducted by Stadel et al4.
- Raje, N. S. et al. Idecabtagene Vicleucel (ide-cel, bb2121) in Relapsed and Refractory Multiple Myeloma (RRMM): Analyses of High-Risk Subgroups in the KarMMa Study. Transplant Cell Ther 27, (2021).
- Munshi, N. C. et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. New England Journal of Medicine 384, (2021).
- Rytlewski, J. et al. Effects of Prior Alkylating Therapies on Preinfusion Patient Characteristics and Starting Material for CAR T Cell Product Manufacturing in Late-Line Multiple Myeloma. Blood 136, (2020).
- Stadel, R. et al. Sequential Administration of Selinexor then CD19 CAR-T Cells Exhibits Enhanced Efficacy in a Mouse Model of Human Non-Hodgkin’s lymphoma. Blood (2022).