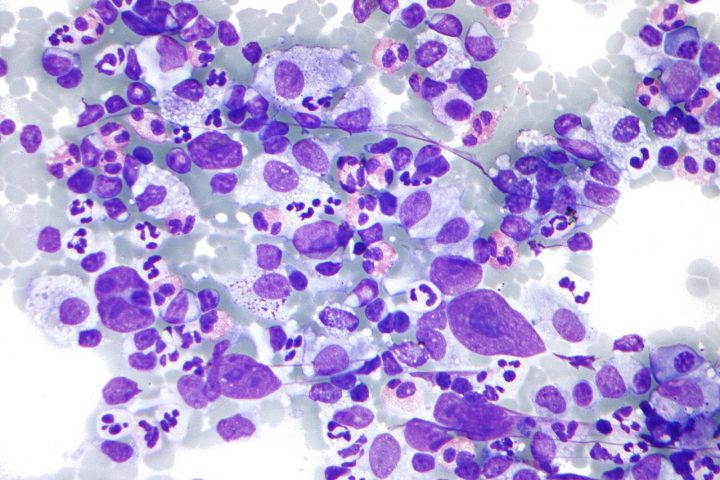
Written by Dr. Chepsy Philip from Believers Church Medical College Hospital, Tiruvalla, Kerala, India KEYNOTE-087 is a multicentre, single-arm, multicohort, nonrandomized, phase 2 study of pembrolizumab in patients with R/R cHL who
On June 20, 2023, the U.S. Food and Drug Administration approved talazoparib (Talzenna, Pfizer, Inc.) in combination with enzalutamide for patients with metastatic castration-resistant prostate cancer (mCRPC) with homologous recombination repair (HRR)
More