By: Dr. Anish Shah Bronx-Lebanon Hospital; Bronx, NY On April 29, 2024, the U.S. Food and Drug Administration granted regular approval to tisotumab vedotin-tftv (Tivdak, Seagen Inc. [now a part of Pfizer Inc.]) for
By: Dr. Anish Shah Bronx-Lebanon Hospital; Bronx, NY On April 23, 2024, the U.S. Food and Drug Administration granted accelerated approval for tovorafenib (Ojemda, Day One Biopharmaceuticals, Inc.) for relapsed or refractory pediatric low-grade
MoreBy: Dr. Anish Shah Bronx-Lebanon Hospital; Bronx, NY On April 29, 2024, the U.S. Food and Drug Administration granted regular approval to tisotumab vedotin-tftv (Tivdak, Seagen Inc. [now a part of Pfizer Inc.]) for
MoreFDA Approves Lutetium Lu 177 Dotatate for SSTR-Positive Gastroenteropancreatic Neuroendocrine Tumors
By: Dr. Anish Shah Bronx-Lebanon Hospital; Bronx, NY On April 23, 2024, the U.S. Food and Drug Administration granted approval for lutetium Lu 177 dotatate (Lutathera, Advanced Accelerator Applications USA, Inc., a Novartis company)
MoreBy: Dr. Anish Shah Bronx-Lebanon Hospital; Bronx, NY On April 18, 2024, the U.S. Food and Drug Administration granted approval to a potent oral ALK (anaplastic lymphoma kinase) tyrosine kinase inhibitor – Alectinib (Alecensa,
MoreBy: Dr. Anish Shah Bronx-Lebanon Hospital; Bronx, NY On April 22, 2024, the U.S. Food and Drug Administration granted approval for nogapendekin alfa inbakicept-pmln (Anktiva, Altor BioScience, LLC), to be used in conjunction with
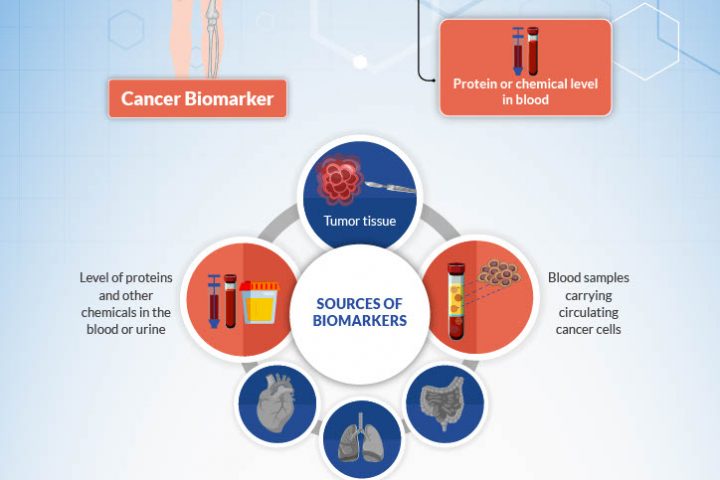
MoreBy Dr. Dipesh Uprety Karmanos Cancer Institute & Dr. Howard (Jack) West City of Hope What is a biomarker, and why do we test for them? A biomarker for cancer
MoreOn June 20, 2023, the U.S. Food and Drug Administration approved talazoparib (Talzenna, Pfizer, Inc.) in combination with enzalutamide for patients with metastatic castration-resistant prostate cancer (mCRPC) with homologous recombination repair (HRR)
MoreOn April 3, 2023, the U.S. Food and Drug Administration approved enfortumab vedontin-ejfv with pembrolizumab for patients with locally advanced or metastatic urothelial carcinoma who have not received prior systemic therapy and
MoreBy Dr. Inas Abuali Massachusetts General Hospital There is a paucity of available treatment options for patients with advanced papillary renal cancer (PRC), a disease characterized by aggressive features and a
MoreOn March 4, 2023, the U.S. Food and Drug Administration approved expanded use of adjuvant abemaciclib with endocrine therapy for adult patients with early, hormone receptor (HR)-positive, human epidermal growth factor receptor
More