On February 3, 2023, the U.S. Food and Drug Administration approved sacituzumab govitecan-hziy for women 18 years or older with HR+/HER2-Metastatic Breast Cancer refractory to or relapsed after at least 2, and
Author: Saramshika Dhakal, MBBS Tribhuvan University Teaching Hospital, Kathmandu, Nepal. In a recent article published in JAMA Oncology (JAMA Oncol. 2023;9(4):465-472; researcher Simiao Chen and the team have estimated that the
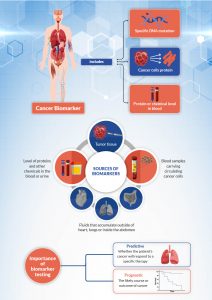
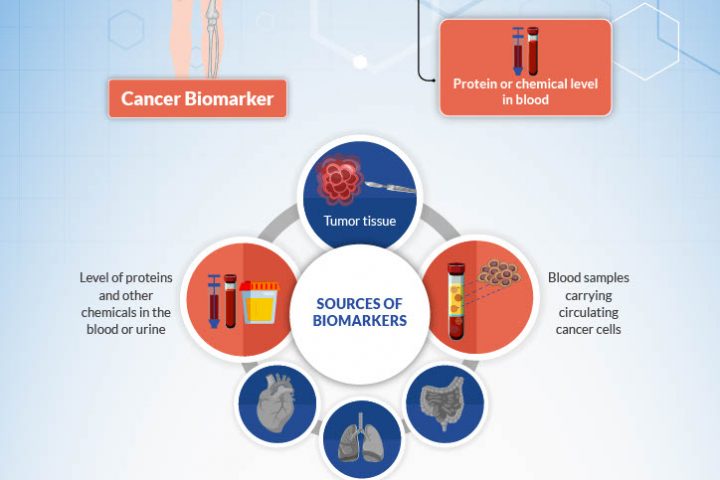
MoreBy Dr. Dipesh Uprety Karmanos Cancer Institute & Dr. Howard (Jack) West City of Hope What is a biomarker, and why do we test for them? A biomarker for cancer
MoreOn July 20, 2023, the U.S. Food and Drug Administration (FDA) approved quizartinib (Vanflyta) for adult patients newly diagnosed with acute myeloid leukemia (AML) that has FLT3 internal tandem duplication (ITD)-positive mutation,
MoreOn March 4, 2023, the U.S. Food and Drug Administration approved expanded use of adjuvant abemaciclib with endocrine therapy for adult patients with early, hormone receptor (HR)-positive, human epidermal growth factor receptor
MoreBy Dr. Richa Parikh Karmanos Cancer Institute & Dr. Harsh Parmar Hackensack Meridian Health A multicenter retrospective study, which was conducted at 11 academic centers across the United States, reported a
MoreBy Dr. Harsh Parmar Hackensack Meridian Health In an unplanned interim analysis of an international phase-III trial (ATLAS) by Dytfeld et al., performed across 12 centers in Poland and the US,
MoreOn February 3, 2023, the U.S. Food and Drug Administration approved sacituzumab govitecan-hziy for women 18 years or older with HR+/HER2-Metastatic Breast Cancer refractory to or relapsed after at least 2, and
MoreDr. Luke Fletcher Willamette Valley Cancer Institute & Research Center Philadelphia positive acute lymphoblastic leukemia treatment has been driven by the combination of tyrosine kinase inhibitors targeting BCR-ABL in conjunction with
MoreDr. Aditi Sharma of Barbara Ann Karmanos Cancer Institute & Dr. Abhishek Mangaonkar of Mayo Clinic In the phase III AGILE trial reported in the New England Journal of Medicine, Pau
MoreThe Foundation Participated in Hackathon by Microsoft to Create Prototype The Binaytara Foundation teamed up with employees of Microsoft to work on the development of our Cancer Crush app in this year’s
More