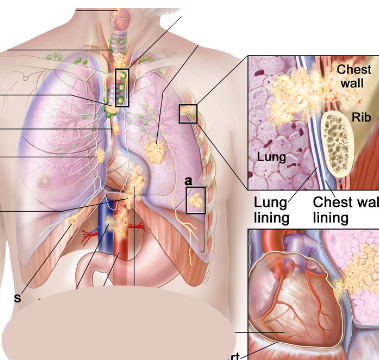
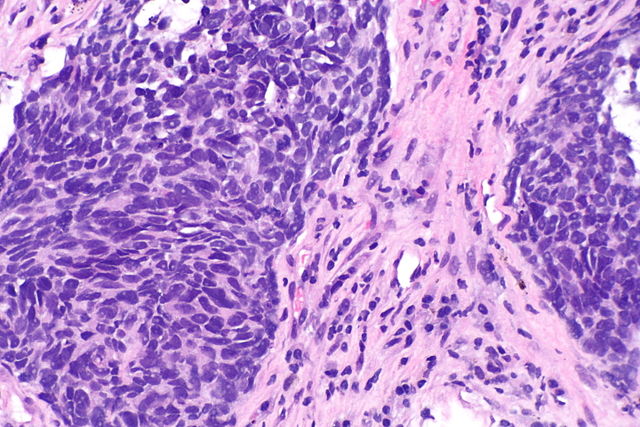
By: Tiantian Zhang1, Yiqun Han2, Lei Deng3 1. Department of Internal Medicine, University of Central Florida, Pensacola, FL 2. Mayo Clinic, Rochester, MN 3. University of Washington/Fred Hutch Cancer Center, Seattle, WA
Bellevue, WA – October 24, 2024 The Binaytara Foundation, a leading global cancer non-profit organization dedicated to improving access to cancer care, is set to host its annual Global Oncology Summit on
MoreOn October 11, 2023, the U.S. Food and Drug Administration granted approval to Encorafenib (BRAF inhibitor) with Binimetinib (MEK inhibitor) for patients with metastatic non-small cell lung cancer (NSCLC) that was detected
MoreAuthor: Saramshika Dhakal, MBBS Tribhuvan University Teaching Hospital, Kathmandu, Nepal. In a recent article published in JAMA Oncology (JAMA Oncol. 2023;9(4):465-472; researcher Simiao Chen and the team have estimated that the
MoreOn September 26, the U.S. Food and Drug Administration expanded the approval of bosutinib (Bosulif) to include pediatric patients 1 year of age and older. Previously, bosutinib had been approved for adults
MorePresented by: Chris Chen, MD, Director, Early Drug Development, Stanford Cancer Institute Covered by: Inas Abuali, MD, FACP Dr. Chris Chen, director of the Early Drug Development Program at Stanford Cancer Institute,
MoreOn August 28, 2023, the U.S. Food and Drug Administration approved luspatercept-aamt (Reblozyl) for the treatment of anemia in adult patients with very low- to intermediate-risk myelodysplastic syndromes (MDS) without prior erythropoiesis-stimulating agent (ESA) use,
More